Search Cidpusa web
IVIG Protocol
😏cidpusa.orgIntroduction IgG
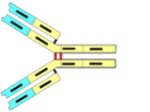
IgG is the main antibody in IVIg that helps full recovery from many disease conditions that are autoimmune like diabetes, CIDP, arthritis, heart disease and alzheimers.
Pediatric AIDS
Because AIDS is an acquired immunodeficiency, it is reasonable to consider the use of IVIG therapy in pediatric patients with HIV infection, given the success of IVIG in primary immunodeficiencies. To date, small, uncontrolled studies have suggested the efficacy of IVIG in pediatric AIDS by decreasing the morbidity from common bacterial pathogens, as well as measles, but no controlled studies have been completed. Currently, the National Institutes of Health (NIH) is sponsoring two large, controlled trials of IVIG with and without zidovudine (AZT) in HIV-infected children. A definitive assessment of the efficacy of IVIG in pediatric HIV infection awaits the results of these trials.
Infections in Low Birth Weight Infants
Prevention of infection in low birth weight infants
Premature infants have insufficient placental transfer of maternal IgG and have been demonstrated to have low levels of serum IgG. Hence, it is reasonable to consider IVIG prophylaxis in premature infants. Some pilot studies indicated a lower rate of severe infection in IVIG-treated low birth weight infants compared with placebo-treated patients. In a recently completed randomized, double-blind, placebo-controlled trial involving a large number of infants with birth weights of 500-1500 grams, it was noted that mortality was not significantly reduced among IVIG recipients. However, the number of infections was significantly reduced among IVIG recipients. It should be noted that there was some evidence that beneficial effect may vary by birth weight category, and significantly more placebo patients were small for gestational age. Information about long-term results (greater than 56 days) is not available from this trial. Moreover, preliminary analysis of other trials indicates no significant difference in infection rates between IVIG recipients and placebo recipients. However, differences in study design make direct comparison of the various trials difficult. Further information will become available as a result of the ongoing trial sponsored by the NICHD Neonatal Research Network. At this time, IVIG cannot be recommended as standard prophylaxis of low birth weight infants.
Treatment of presumed neonatal infection
To date, small trials using primarily historical controls have yielded mixed results. Questions remain concerning dose, schedule, and patient selection. Variability in preparations and lots creates a number of difficulties in predicting results of treatment for specific organisms. There is a potential role for directed preparations containing specific antibodies. Furthermore, studies in neonatal animals have shown that in some situations survival is less with high concentrations of IVIG plus antibiotic compared with antibiotic alone. The routine use of IVIG as adjuvant therapy of neonatal infections cannot be recommended at the present time.
Bone Marrow Transplantation
In a large study of bone marrow transplant recipients, reduced rates of septicemia and local infection were noted for IVIG-treated patients (500 mg/kg weekly) compared with untreated controls. Several studies have shown a decreased rate of acute graft versus host disease (GVHD) in patients receiving IVIG, 500 milligrams to 1 gram per kilogram weekly. Results of limited trials suggest that in pediatric bone marrow recipients, reduction in infection and death but not GVHD has been associated with IVIG administration.
It has been noted that IVIG decreases the incidence of interstitial (presumably CMV) pneumonia but is ineffective in preventing CMV infections. IVIG plus ganciclovir is beneficial in treating CMV pneumonia in patients who are not ventilator dependent.
Chronic Lymphocytic Leukemia
A study in 10 centers in which 57 patients with CLL were followed for 1 year of observation has been completed. The incidence of major and moderate bacterial infections was significantly reduced in hypogammaglobulinemic CLL patients who received IVIG. The number of trivial infections was unchanged by intravenous immunoglobulin. Maintenance of serum IgG levels > 640 mg/dL tended to correlate with fewer infections, especially serious bacterial infections. The data support the conclusion that IVIG may be useful to prevent serious infections in patients with CLL with hypogammaglobulinemia.
Idiopathic Thrombocytopenic Purpura
Among children with ITP, the use of IVIG has been documented to increase platelet counts. This treatment is utilized in the rare pediatric patient with potentially life-threatening bleeding (e.g., 400 mg/kg/d x2-5 or 1 g/kg/d x1 or 2). A number of therapeutic options are available for managing the child with newly diagnosed ITP who does not have serious hemorrhage: IVIG, corticosteroids, or close observation without therapy. IVIG has been used in chronic ITP to postpone the requirement for a splenectomy. There is no firm evidence of curative effects in either acute or chronic ITP. Responses appear to be similar with different manufacturers' products licensed for this purpose.
In adult patients with ITP, IVIG, at the doses indicated above, has also been used for the rapid correction of life-threatening thrombocytopenia, with or without corticosteroids. Administration every 10 to 21 days is usually required to maintain adequate platelet counts. Other indications include administration to steroid-refractory patients preoperatively. In addition, IVIG may be employed at the same dosage in patients who cannot use corticosteroids and in patients with immunodeficiency including those with HIV-associated thrombocytopenia. Although IVIG administration before scheduled splenectomy is effective, cost/benefit relationships are unclear because of the low incidence of complications during this procedure, even with extremely low platelet counts.
Kawasaki Syndrome
Studies of IVIG in Kawasaki syndrome using 400 mg/kg daily for 4 days indicate a prompt anti-inflammatory response in the acute phase and a significant decrease in the formation of coronary aneurysms compared with low/moderate aspirin administration regimens. More recently, a dosage schedule of 2 g/kg as a single administration has been shown to be at least as effective as the four-dose schedule. Complications of the larger single-dose administration were few. A smaller study using 1 g/kg as a single dose also seemed to suggest similar efficacy to the four-dose regimen.
IVIG and aspirin administration has become a standard treatment for Kawasaki syndrome. The panel agrees that on the basis of available studies, IVIG administered as a single dose of 2 g/kg is effective therapy for patients who fulfill the diagnostic criteria for Kawasaki syndrome. Treatment of patients recognized after the tenth day of the disease has not been studied systematically.
The advantages of treating patients with Kawasaki syndrome with IVIG as early as possible must be balanced against the probability that children with other inflammatory diseases will also be treated inadvertently. There is no evidence that this latter group of children will be benefitted.
Chronic Inflammatory Demyelinating Polyneuropathies
Several small studies have shown positive responses to IVIG in the majority of treated patients. Treatment needs to be periodically repeated to prevent relapse. When considered in comparison with customary treatments, IVIG may be easier to use and associated with fewer complications than repeated therapeutic plasma exchange and long-term glucocorticoids, respectively.
Guillain-Barré Syndrome
In a preliminary analysis of a large, randomized multicenter trial of IVIG compared with therapeutic plasma exchange, there is an indication of improved functional recovery for patients receiving IVIG. Definitive conclusions regarding the efficacy of IVIG in Guillain-Barré syndrome will need to await the final analysis of this study and other confirmatory studies.
Intractable Seizure Disorders
A number of uncontrolled studies and anecdotal reports have indicated benefit of IVIG administration in children with intractable seizures. The absence of randomized, double-blind studies does not allow specific recommendations to be made regarding such treatment. The panel emphasizes the need for controlled human studies in this area.
Continue to next page of Handbook of polyneuropathy😏